
"Humoral immunity, immunobiotechnology" research group
„Humoral immunity, immunobiotechnology” research group
(„FcRn and antibody” research group)
Head of the research groupImre Kacskovics (DVM., Ph.D., D.Sc., Professor, Peter S. Freudenthal professor) Members of the research group
|
Research interest
Immunobiotechnology
The production of recombinant antibodies from single isolated B cells is based on the isolation of single B cells, the amplification of the immunoglobulin heavy and light chains of these isolated B cells and co-expression in mammalian expression cells with the help of constant region containing vectors.
Our aim is to further improve this methodology therefore we used the COVID-19 as a model antigen to produce antigen specific human monoclonal recombinant antibodies in a more efficient and faster way.
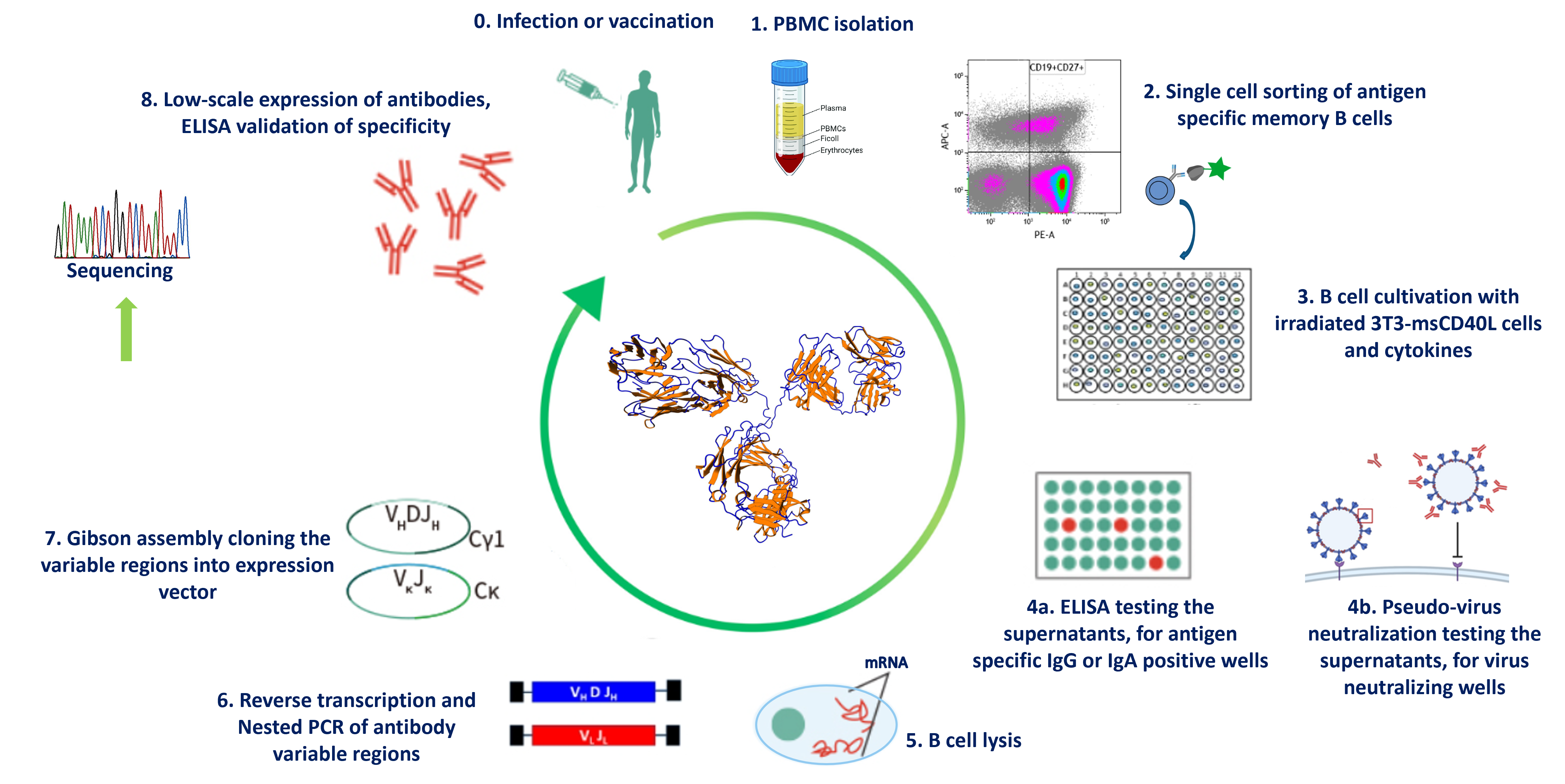
Schematic overview of the main steps of human monoclonal antibody production
Single SARS-CoV-2 specific memory B cells were sorted out with the help of fluorescence-activated cell sorting (FACS) after the isolation of PBMCs from the blood of people who were vaccinated or recovered from the disease. To expand the sorted memory B cells, a feeder cell layer of irradiated 3T3-msCD40L cells and some additional cytokines were used. In this environment, the B cells were able to proliferate and produce a small amount of antibodies. We used the supernatant of the B cell clones and tested them whether they can recognize the target or not. The positive samples were used to produce recombinant antibodies from them.
In the case of the SARS-CoV-2 specific human monoclonal antibodies a pseudo virus neutralization test (pVNT) was performed to test the functional abilities of the antibodies. This test can tell us whether these antibodies can inhibit the virus infection or not and it can be used for the testing the presence of neutralizing antibodies in the sera of individuals.
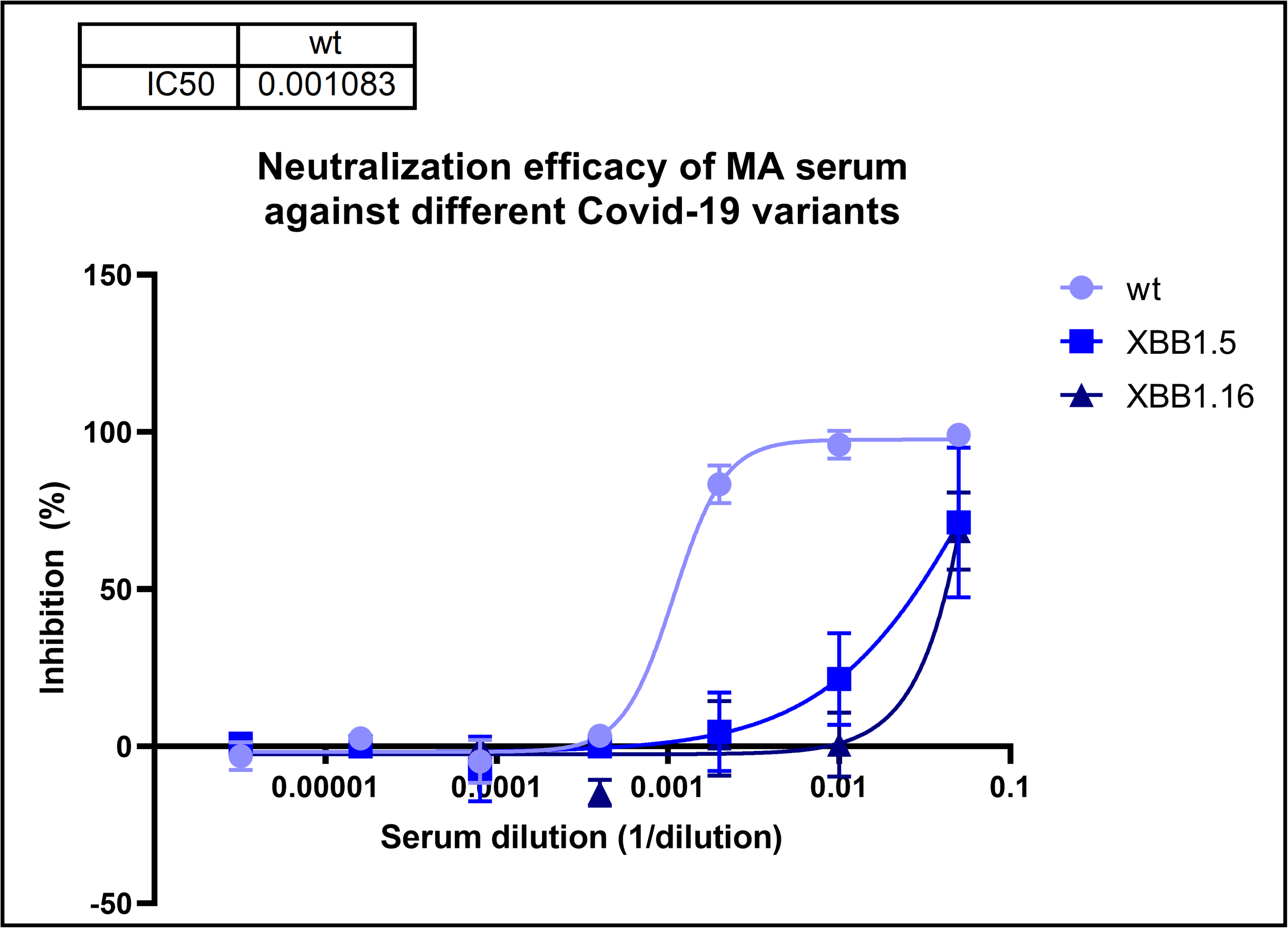
pVNT assay: Neutralization efficacy of a serum from a donor
This methodology can be used to produce human monoclonal antibody against any other target and can be useful to characterize autoantibodies as well.
FcRn
Over the last two decades therapeutic and diagnostic uses of antibodies have been greatly progressed. However, producing antibodies that are specific for the current, most promising therapeutic targets is highly challenging.
The neonatal Fc receptor (FcRn) regulates IgG and albumin homeostasis, mediates maternal IgG transport, takes active part in phagocytosis and delivers antigen for presentation.
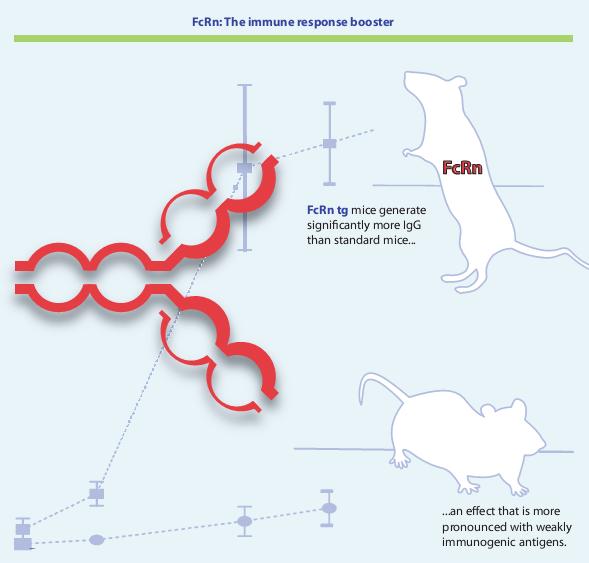
Enhanced FcRn expression augments antigen specific IgG titers and antigen specific B cell numbers, especially in case of weakly immunogenic targets.
We have created transgenic mice and rabbits that overexpress FcRn and show significantly improved humoral immune responses with:
1) higher antigen specific serum IgG titers;
2) larger numbers of antigen specific B cells in the spleen offering more target specific hybridomas;
3) increased diversity of induced antibodies;
4) generation of antibodies against weakly immunogenic antigens.
Therefore, these animals allow the production of high quality and diverse antibodies for challenging therapeutic, diagnostic and research targets.
We have filed patent applications to protect this innovation that lead to issued patents in the European Union, Hong Kong and Australia (EP2097444, HK1134305, and AU2007323049, respectively) so far, while patent applications are pending in other major regions (USA, Canada, Japan and China) and founded a start-up company ImmunoGenes to transfer this technology to the industry. Our transgenic animals have been tested for their effectiveness at the world’s largest pharma companies (e.g. AMGEN, Bristol-Myers Squibb) which may integrate them into their antibody development technologies. ImmunoGenes has recently initiated its premium category “Customized Antibody Solution” program to develop monoclonal- and polyclonal-antibodies against challenging targets.
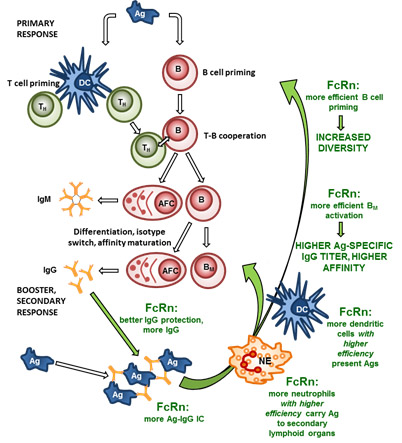
FcRn overexpression rescues IgG more efficiently resulting in higher level of antigen-specific IgGs in immunized transgenic animals which leads to the formation of more antigen-IgG ICs. Transgenic dendritic cells (DC) that overexpress FcRn present antigens more efficiently to T helper cells (TH) when loaded with antigen-IgG ICs. It also enhances the priming of naïve B cells, the expansion of antigen-specific memory B cells (BM) and plasma cells (antibody forming cells; AFC) in the secondary lymphoid organs. This results in a more diverse humoral immune response, a higher titer and higher affinity of antigen-specific IgG. (Green texts and arrows indicate cells and effects that contribute in augmenting the humoral immune response by FcRn.)
Research projects
- Nemzeti Laboratórium létrehozása, komplex fejlesztése: Nemzeti Gyógyszerkutatási és Fejlesztési Laboratórium (PharmaLab) (RRF-2.3.1-21-2022-00015).
2022.03.01 - [2026.02.28.]
The project number RRF-2.3.1-21-2022-00015 was implemented with the support of the European Union in the frame of the Széchenyi Plan Plus
 |
 |
Further projects
- Mielóma multiplex MRD elemzését lehetővé tévő NGS elemzés laboratóriumi szintű megvalósítása, ELTE Felsőoktatási Intézményi Kiválósági Program, Diagnosztika és Terápia projekt, Holobiont és Diagnosztika alprojekt (1783-3/2018/FEKUTSRAT, majd NKFIH-1157-8/2019-DT)
2018 - 2021.
Emberi Erőforrások Minisztériuma, majd a Nemzeti Kutatási Fejlesztési és Innovációs Hivatal támogatásával
 |
 |
- Biologikumok gyártástechnológiájának optimalizálása és az azt támogató analitikai vizsgálati módszerek fejlesztése (2017-1.3.1-VKE-2017-00002)
2018.02.01. – 2021. 01.31.
Konzorciumi tag
Nemzeti Kutatási Fejlesztési és Innovációs Alapból biztosított támogatással

- Áramlási citofluoriméter alkalmazása terápiás, diagnosztikai és kutatási ellenanyagok fejlesztésére és elemzésére (VEKOP-2.3.3-15-2017-00021)
2017.06.01 - 2020.05.31.
Támogatja: Széchenyi2020
További információk: ELTE Pályázati Központ weboldal

- Molekuláris biomarker kutatási és szolgáltatási központ kialakítása az ipari igények kiszolgálása érdekében, ELTE Biotechnológia Felsőoktatási és Ipari Együttműködési Központ (FIEK_16-1-2016-0005)
2017.04.01 - 2021.03.31.
Konzorciumi tag
Nemzeti Kutatási Fejlesztési és Innovációs Alapból biztosított támogatással
ELTE Biotechnológia FIEK weboldal

- Bioszimiláris monoklonális antitestek fejlesztése (VKSZ_12-1-2013-0001)
2014.01.01 - 2017.12.31.
Konzorciumi tag
Támogatja: Széchenyi2020

- Analysis of the basic mechanisms of the humoral immune response in transgenic mice that overexpress the neonatal Fc receptor (OTKA K101364)
2012 - 2014.
Nemzeti Kutatási Fejlesztési és Innovációs Hivatal támogatásával


